Melphalan and the Liver: Understanding Hepatic Toxicity and Management Strategies
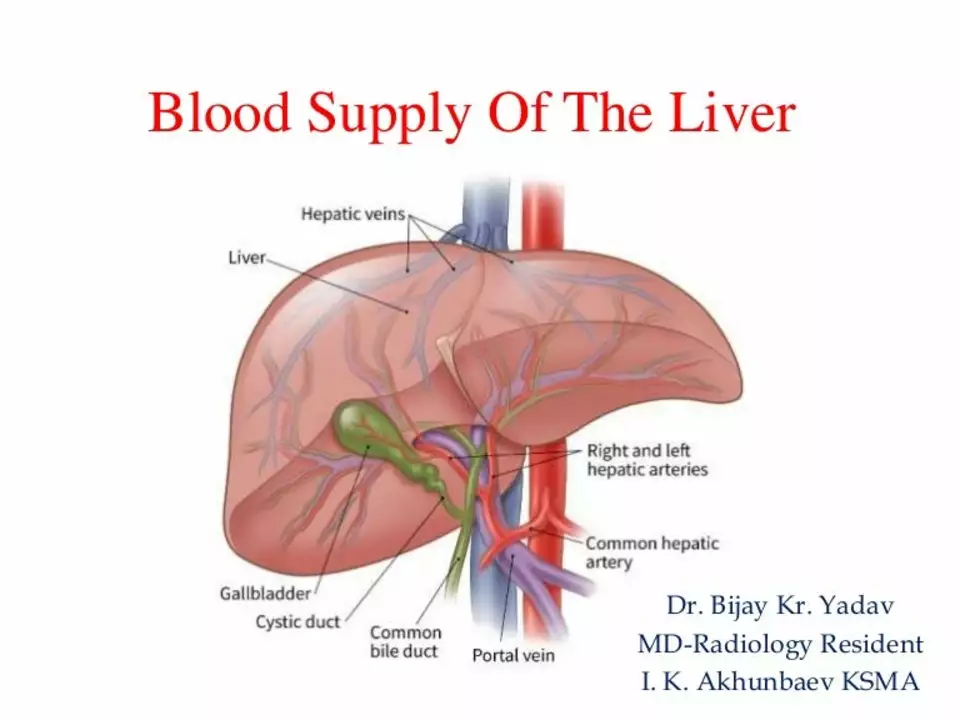
Introduction to Melphalan and Hepatic Toxicity
As a healthcare blogger, I find it essential to discuss Melphalan and its potential adverse effects on the liver. Melphalan is a chemotherapy drug used to treat various cancers such as multiple myeloma, ovarian cancer, and breast cancer. However, like many other chemotherapy drugs, Melphalan can cause hepatic toxicity or damage to the liver. In this article, I will provide an overview of hepatic toxicity and discuss different management strategies to minimize the risk of liver damage associated with Melphalan use.
Understanding the Role of the Liver in Drug Metabolism
The liver plays a crucial role in the metabolism of drugs, including Melphalan. Once the drug enters the bloodstream, it is metabolized by the liver into its active form, which can then exert its therapeutic effects on cancer cells. However, the liver can also be exposed to toxic byproducts generated from the drug's metabolism, leading to hepatic toxicity. Understanding the role of the liver in drug metabolism is crucial for developing appropriate management strategies to prevent or mitigate hepatic toxicity.
Factors Contributing to Melphalan-Induced Hepatic Toxicity
Several factors contribute to the development of hepatic toxicity in patients receiving Melphalan. These include the drug's dose, duration of treatment, patient's age, and pre-existing liver conditions. Additionally, genetic factors may also predispose certain individuals to develop liver damage in response to Melphalan. It is essential to be aware of these factors and consider them when prescribing Melphalan to minimize the risk of hepatic toxicity.
Signs and Symptoms of Hepatic Toxicity
Early detection of hepatic toxicity is crucial for implementing appropriate intervention strategies. Some common signs and symptoms of liver damage include jaundice (yellowing of the skin and eyes), fatigue, nausea, vomiting, abdominal pain, and dark urine. It is essential to educate patients receiving Melphalan about these signs and symptoms and encourage them to report any concerns promptly to their healthcare provider.
Monitoring Liver Function during Melphalan Treatment
Regular monitoring of liver function is crucial for early detection and management of hepatic toxicity in patients receiving Melphalan. Healthcare providers typically perform blood tests, such as liver function tests (LFTs), to assess the liver's ability to process and eliminate the drug. These tests help identify any potential liver damage and allow for timely intervention, such as dose modification or discontinuation of the drug.
Management Strategies for Melphalan-Induced Hepatic Toxicity
Several management strategies can be implemented to minimize the risk of hepatic toxicity in patients receiving Melphalan. These include:
Dose Modification
Adjusting the dose of Melphalan can help reduce the risk of liver damage. Healthcare providers may consider lowering the dose or extending the duration between doses, especially in patients with pre-existing liver conditions or those who have experienced previous episodes of hepatic toxicity.
Drug Discontinuation
In some cases, discontinuing Melphalan may be necessary to prevent further liver damage. This decision is typically made based on the severity of hepatic toxicity and the patient's overall health status.
Supportive Care
Supportive care measures, such as hydration and nutrition, can help minimize the risk of hepatic toxicity. Ensuring that patients receive adequate fluids and maintain a balanced diet can support liver function during Melphalan treatment.
Use of Hepatoprotective Agents
Some medications can help protect the liver from damage caused by Melphalan. These hepatoprotective agents can be prescribed on a case-by-case basis, depending on the patient's overall health status and the risk of hepatic toxicity.
Conclusion
Melphalan is a valuable chemotherapy drug used to treat various cancers, but its use can also lead to hepatic toxicity. Understanding the factors contributing to liver damage, recognizing the signs and symptoms of hepatic toxicity, and implementing appropriate management strategies can help minimize the risk of liver injury in patients receiving Melphalan. As a healthcare blogger, I hope this article has provided valuable information for both healthcare providers and patients to better understand and manage Melphalan-induced hepatic toxicity.
17 Comments
andrew bigdick
When you’re considering melphalan dosing, it helps to look at the patient’s baseline liver enzymes and adjust incrementally. A lower starting dose can give the liver a breather, especially for those with pre‑existing steatosis. It’s also wise to schedule LFTs every couple of weeks during the first cycle – catching any uptick early can prevent a full‑blown toxicity. If you notice a rise in ALT or AST, a short break or dose reduction often does the trick. The key is keeping communication open with the oncology team and the patient’s primary doc, so everyone’s on the same page about modifications.
Shelby Wright
Oh, the drama of “just a little liver bump” – as if we’re sipping tea while the organ is on fire! Let’s be real: melphalan can turn your liver into a warzone if you ignore the red flags. I’d say the real toxic villain here is complacency, not the chemo itself. So, skip the sugar‑coated “mild side effects” and actually watch those bilirubin levels like a hawk. Trust me, some patients will thank you when you catch the trouble before it turns into a full‑blown hepatic nightmare.
Ellen Laird
One must not forgo the subtle witticisms of hepatic physiology whilst discoursing upon chemotherapeutic agents. It is, indeed, quite memborable that melphalin, albeit efficent, may harbinger side‑effects that are oft underappreciated. The predominate emphasis should be on a robust monitoriing regimen, lest the pretentious notion of “acceptable toxicity” be MISTAKEN. Furthermore, the genertic predispositions cannot be overlooked... albeit I occasionally type wass certain things incorrectly.
rafaat pronoy
Got to love the balance between chemo and a good hydration plan 😊. Keeping fluids up while on melphalan helps the liver flush out toxins faster. Also, a light snack before the infusion can stave off nausea, which indirectly protects the liver from stress. Don’t forget the little things – a short walk after treatment can boost circulation and aid liver function. Cheers to staying proactive and keeping those labs in the green!
sachin shinde
While the previous comment exudes a carefree tone, it neglects precise terminology. It is “hydration”, not “hydrations”, and “chemo” should be capitalized as “Chemotherapy” in formal discourse. Moreover, suggesting a “short walk” without considering the patient’s overall condition borders on irresponsible. An accurate assessment requires a thorough review of hepatic function tests before prescribing any adjunctive activity. Please ensure your recommendations are evidence‑based and grammatically sound.
Leon Wood
Hey team, let’s keep the morale high! Even when melphalan throws a curveball at the liver, we’ve got strategies to dodge it. Think of dose tweaks as a game of chess – every move counts, but there’s always a winning plan. Encourage patients to stay hydrated, stay positive, and keep those follow‑up labs on schedule. Together we can turn a potential setback into a success story.
George Embaid
Your optimism is refreshing, and I’d add that cultural sensitivity matters too. Some patients may have dietary customs that affect liver health, such as avoiding certain herbs during chemotherapy. Tailoring supportive care to respect those practices can improve adherence to hydration and nutrition recommendations. In turn, that respect can enhance overall outcomes and patient satisfaction.
Meg Mackenzie
Honestly, the pharma industry doesn’t want you to know how easily melphalan can sabotage your liver. They push the drug, downplay the toxicity, and rely on half‑hearted monitoring protocols. It’s a classic case of profit over patient safety. Stay vigilant, demand full transparency, and never trust a blanket statement that “side effects are manageable”.
Shivaraj Karigoudar
When delving into the multifaceted mechanisms by which melphalan exerts hepatotoxic effects, one must first acknowledge the intricacies of phase I and phase II metabolic pathways that are predominantly orchestrated by hepatic microsomal enzymes. The alkylating nature of melphalan leads to the formation of DNA adducts not only in neoplastic cells but also in hepatocytes, precipitating a cascade of cellular stress responses.
Subsequent activation of the oxidative stress axis engenders a surplus of reactive oxygen species (ROS), which can overwhelm endogenous antioxidant defenses such as glutathione peroxidase and superoxide dismutase.
Concomitantly, the upregulation of pro‑inflammatory cytokines, notably TNF‑α and IL‑6, amplifies the inflammatory milieu, fostering hepatocellular apoptosis and necrosis.
In patients harboring polymorphisms in genes encoding for glutathione S‑transferases, the detoxification capacity is further compromised, rendering the liver more susceptible to injury.
Clinical manifestations may range from asymptomatic transaminase elevations to overt cholestasis, underscoring the necessity for vigilant monitoring.
Serial measurement of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and bilirubin should be instituted prior to each treatment cycle.
Moreover, integrating non‑invasive imaging modalities such as transient elastography can provide adjunctive insight into early fibrotic changes.
Therapeutically, dose modulation remains a cornerstone; however, adjunctive prophylactic agents like N‑acetylcysteine have shown promise in attenuating oxidative damage, albeit data remain preliminary.
Hydration protocols, tailored to individual renal function, aid in renal clearance of melphalan and its metabolites, indirectly mitigating hepatic overload.
Nutritionists should emphasize a diet rich in antioxidants – berries, leafy greens, and omega‑3 fatty acids – to bolster endogenous defenses.
In cases of significant LFT derangement, temporary cessation of melphalan coupled with hepatoprotective agents such as silymarin may be warranted.
Interdisciplinary collaboration among oncologists, hepatologists, and pharmacists ensures a balanced risk‑benefit assessment.
Future research should focus on pharmacogenomic profiling to predict susceptibility and guide personalized dosing regimens.
In summary, an integrative approach encompassing vigilant surveillance, supportive care, and potential pharmacologic interventions can substantially curtail melphalan‑induced hepatic toxicity.
Matt Miller
Regular liver function tests are essential during melphalan therapy.
Fabio Max
Absolutely, keeping those labs in check lets us catch problems early and tweak the dose before anything serious develops.
Darrell Wardsteele
The guideline for monitoring is crystal clear – you must not skip any scheduled LFT. Any deviation is a sign of weak protocol adherence which is unacceptable. Also, the suggestion to “stay hydrated” is trite; the focus should be on strict fluid balance calculations and evidence‑based dosing.
Madeline Leech
Let’s be honest, these so‑called “evidence‑based” protocols are often just a cover for corporate profit motives. Real doctors know that the system pushes you to minimize costs, not maximize patient safety. We need to call out the complacency and demand true transparency.
Barry White Jr
Good point.
Andrea Rivarola
I appreciate the brevity, but let me elaborate: while succinct affirmations are welcome, the nuances of hepatic monitoring deserve a more thorough discussion. It is crucial to underscore the importance of baseline assessments, patient education on symptom recognition, and the interdisciplinary coordination that ensures timely interventions. Each of these components plays a vital role in safeguarding liver health during melphalan therapy.
Tristan Francis
Simple fact: melphalan can hurt the liver if you don’t watch the labs.
Keelan Walker
Exactly! 📊 Monitoring is the unsung hero of chemotherapy safety. By keeping a close eye on liver enzymes, we can intervene before damage becomes irreversible. It’s like having a radar that spots storms early – you adjust your course and keep the crew safe. 🌟 Let’s make it a habit to review LFTs before every dose and involve patients in the conversation so they feel empowered. Together, we’ll turn potential toxicity into a manageable side effect. 🚀





Write a comment