Pharmacogenomics Testing: How Your Genes Decide Which Medications Work for You
Pharmacogenomics Medication Checker
Enter a medication to see how your genes might affect your response. Based on CPIC guidelines and FDA-approved drug labels.
Enter a medication name to see how your genes might affect your response to this drug.
Imagine taking a pill that doesn’t work-again. And again. And again. You’ve tried five antidepressants, each one leaving you tired, nauseous, or worse. Your doctor says, "It’s just trial and error." But what if the problem isn’t you? What if it’s your genes?
That’s where pharmacogenomics testing comes in. It’s not science fiction. It’s happening right now in hospitals and clinics across the U.S. and Europe. This isn’t about predicting disease. It’s about predicting how your body will react to the drugs doctors prescribe. Your DNA holds clues that can tell your doctor whether a medication will help you, hurt you, or do nothing at all.
Why Your Genes Matter More Than Your Symptoms
For decades, doctors treated patients based on symptoms, age, weight, and sometimes gender. But two people with identical depression symptoms can respond completely differently to the same SSRI. One gets better. The other gets worse. Why? Because their bodies process the drug differently-thanks to variations in genes like CYP2D6, CYP2C19, and CYP2C9. These genes code for liver enzymes that break down about 75% of all prescription medications.
Some people are fast metabolizers. Their bodies clear drugs too quickly, so the medication never builds up enough to work. Others are slow metabolizers. The drug sticks around too long, building up to toxic levels. A simple blood or saliva test can tell you which group you’re in. For example, if you’re a poor metabolizer of CYP2D6, common antidepressants like fluoxetine or paroxetine could make you dizzy, nauseous, or even trigger suicidal thoughts. But switch to bupropion, which doesn’t rely on that enzyme, and you might feel better within days.
The data backs this up. A 2022 meta-analysis in the Journal of Clinical Psychiatry found that patients who got treatment guided by pharmacogenomics were 30.5% more likely to achieve remission than those on standard care. That’s not a small improvement. That’s life-changing.
Real-World Cases: When Genes Saved Lives
One of the clearest success stories is with abacavir, an HIV drug. Before pharmacogenomics, about 5-8% of patients developed a deadly hypersensitivity reaction-fever, rash, breathing trouble. It was unpredictable. Then doctors learned that people carrying the HLA-B*57:01 gene variant had a 50-60% chance of that reaction. Now, every patient gets tested before starting abacavir. The reaction rate? Nearly zero. The FDA now requires this test. It’s a black box warning with a simple fix: test first, then prescribe.
Another example is clopidogrel (Plavix), a blood thinner used after heart attacks. About 30% of people have a CYP2C19 variant that turns the drug into an inactive form. For them, Plavix might as well be sugar pills. The result? Higher risk of heart attack or stroke. But if you know you’re a poor metabolizer, your doctor can switch you to prasugrel or ticagrelor-drugs that don’t need that enzyme. Studies show this cuts major cardiac events by half.
And then there’s tamoxifen, used for breast cancer. It needs CYP2D6 to become active. If you’re a poor metabolizer, the drug doesn’t work. Your cancer keeps growing. Testing before starting treatment can save lives.
What Gets Tested? The Top 5 Gene-Drug Pairs
Not every drug needs genetic testing. But for some, it’s critical. Here are the most clinically important gene-drug pairs right now:
- CYP2D6 + antidepressants (SSRIs, SNRIs), opioids (codeine, tramadol): Determines if you’ll get relief or side effects.
- CYP2C19 + clopidogrel, proton pump inhibitors (omeprazole): Affects heart drug efficacy and stomach acid control.
- CYP2C9 + warfarin (blood thinner): Helps find the right dose to prevent clots without bleeding.
- HLA-B*57:01 + abacavir: Prevents life-threatening allergic reactions.
- TPMT + thiopurines (azathioprine, mercaptopurine): Prevents severe bone marrow suppression in leukemia and autoimmune patients.
The FDA lists 178 drugs with pharmacogenomic info in their labels. That number is growing fast. In 2000, only 10% of new drugs included this data. By 2022, it was 28%. The trend is clear: drugmakers are building genetics into their designs.
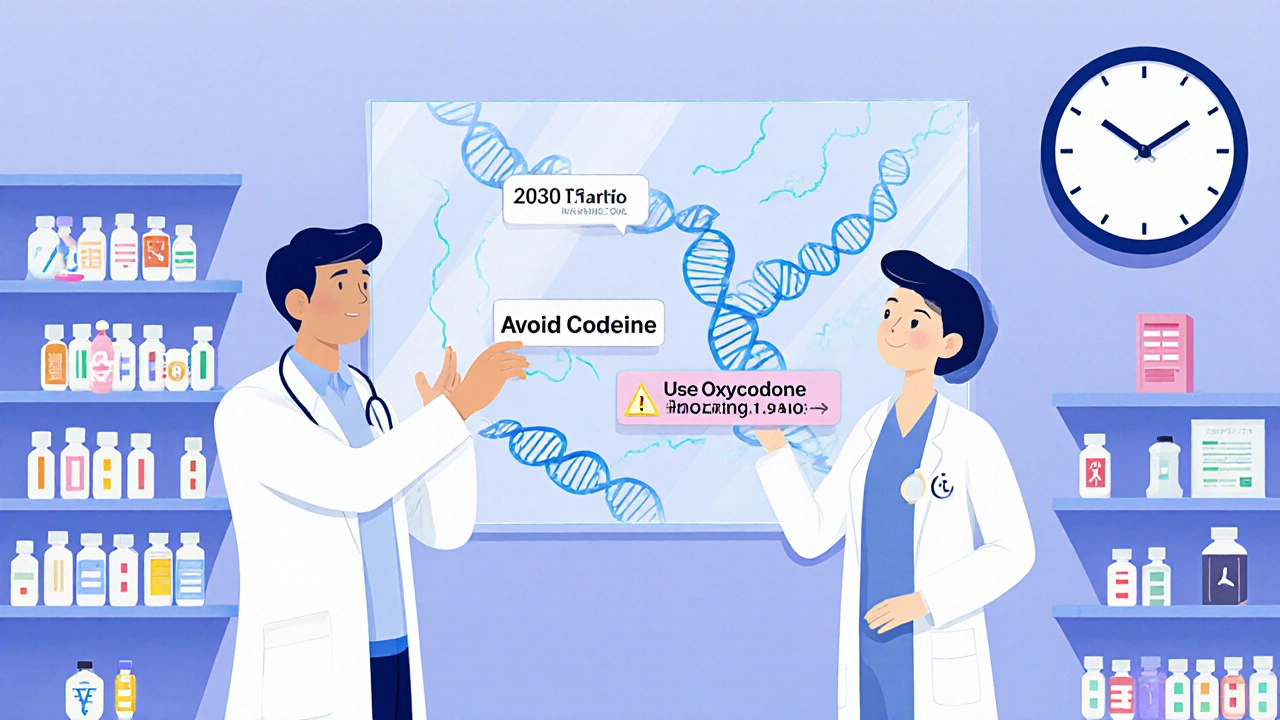
How It Works: From Saliva to Prescription
Getting tested is simpler than you think. You spit into a tube or get a quick cheek swab. No needles, no fasting. The sample goes to a CLIA-certified lab-companies like OneOme, Invitae, or Genelex handle most of these tests. Results come back in 3 to 14 days.
The report doesn’t just say “you’re a slow metabolizer.” It tells your doctor exactly what to do. For example:
- “Avoid codeine. Use oxycodone instead.”
- “Start escitalopram at half dose.”
- “Consider alternative to clopidogrel.”
These recommendations come from the Clinical Pharmacogenetics Implementation Consortium (CPIC), a group of experts that reviews global evidence and publishes clear guidelines. Their rules are used by hospitals, EHR systems like Epic, and even pharmacy benefit managers.
Some systems now auto-flag drug-gene conflicts. If your doctor tries to prescribe warfarin to someone with a CYP2C9 variant, the system pops up: “Genetic test suggests lower starting dose.” It’s not magic. It’s smart software using real science.
Limitations: It’s Not a Crystal Ball
Pharmacogenomics isn’t perfect. It doesn’t explain everything. Studies show genes account for only 10-15% of why people respond differently to drugs. Other factors matter too: age, liver function, other medications, diet, even gut bacteria.
Also, most testing today focuses on a few key genes. But there are thousands of genes involved in drug response. Right now, we only understand the big ones. And here’s a big problem: most research has been done on people of European descent. The data for African, Asian, or Indigenous populations is still limited. That means the test might be less accurate for non-European patients.
And not every drug needs it. Penicillin? No need. Ibuprofen? Probably not. Pharmacogenomics shines where the stakes are high-psychiatry, oncology, cardiology, pain management. For those, it’s worth it. For others? It’s overkill.
Cost, Coverage, and Access
A targeted pharmacogenomics test costs between $250 and $500. Whole genome sequencing runs $1,000-$2,000. Most clinics use the targeted panels-they’re cheaper and focused on what matters.
Insurance coverage? It’s patchy. Medicare covers testing for specific cases like clopidogrel or warfarin. Some private insurers cover it for depression or cancer drugs. But only 35% of commercial plans cover it broadly, according to a 2023 survey by the American Pharmacists Association. Many patients pay out of pocket.
Still, the cost of not testing is higher. Adverse drug reactions cause over 2 million hospitalizations and 100,000 deaths in the U.S. every year. One study estimated that widespread pharmacogenomics use could save the healthcare system $137 billion annually.
Who’s Using It? And Who’s Left Behind?
Academic medical centers are leading the charge. 63% of them have pharmacogenomics programs. Community hospitals? Only 17%. Why? Training. Most doctors didn’t learn genetics in med school. A 2022 survey found only 15% of physicians feel confident interpreting results.
Pharmacists are stepping in. 72% of academic hospitals now have pharmacogenomics-trained pharmacists who help interpret reports and adjust prescriptions. They’re the bridge between the test and the patient.
Patients report high satisfaction. On Healthgrades, pharmacogenomics services average 4.1 out of 5 stars. But 42% say their doctors didn’t know what to do with the results. That’s the biggest hurdle-not the test, but the system.
The Future: Routine Genetic Profiles in Your Medical Record
Right now, you get tested for one drug at a time. But the future is pre-emptive testing: one test, lifelong results. Your DNA is stored in your EHR. Every time a new drug is prescribed, the system checks your profile automatically.
The NIH’s eMERGE network is already doing this in 100,000 patients. The All of Us program has genomic data on over 620,000 people-many with pharmacogenomic results now being returned to participants. By 2030, experts predict half of all U.S. adults will have their pharmacogenomic data in their medical records.
Point-of-care tests are coming too. Imagine a doctor’s office running a cheek swab during your visit and getting results in 2 hours. That’s already in FDA trials.
And it’s not just drugs. Future tests may predict how you’ll respond to vaccines, anesthesia, or even chemotherapy based on your genetic profile. This isn’t the future. It’s the next five years.
What Should You Do?
If you’ve struggled with medications-especially antidepressants, painkillers, or heart drugs-ask your doctor about pharmacogenomics testing. Don’t wait for them to bring it up. Be proactive.
Ask:
- “Have you heard of pharmacogenomics testing?”
- “Could my genes be affecting how I respond to my meds?”
- “Is there a test that could help avoid more trial and error?”
Bring up your history: “I’ve tried five antidepressants. None worked.” That’s the exact scenario where this test helps the most.
If your doctor says no, ask for a referral to a pharmacist specializing in pharmacogenomics. Many academic hospitals have them. Some telehealth services offer testing with provider support.
This isn’t about replacing your doctor. It’s about giving them better tools. You’re not a guessing game. You’re a person with a unique biology. Your genes are part of your medical story. It’s time they were heard.
14 Comments
Matthew Kwiecinski
Pharmacogenomics isn’t new, but it’s finally getting the attention it deserves. The CYP450 system has been studied since the 80s, and we’ve known for decades that metabolism variations affect drug response. What’s changed? The cost of sequencing dropped and EHRs got smart enough to flag conflicts. That’s it. No magic. Just data catching up to science.
Jens Petersen
Let’s be real-this isn’t precision medicine. It’s precision marketing. Pharma companies love this because it lets them sell more expensive tests while sticking patients with the bill. And don’t get me started on the racial bias in the data. Most of these gene-dosage guidelines were built on white, middle-class populations. If you’re not European, your results might as well be a horoscope. They call it science. I call it arrogance wrapped in a lab coat.
Keerthi Kumar
As someone from India, where polypharmacy is common and access to genetic testing is nearly nonexistent, I find this both inspiring and heartbreaking. We have millions who suffer from antidepressant inefficacy or severe side effects, yet we lack the infrastructure to even test for CYP2D6 variants. The knowledge exists, but equity doesn’t. We need global partnerships-not just for testing, but for training local pharmacists to interpret results. This isn’t just a medical issue. It’s a justice issue.
Dade Hughston
so i got tested last year after 7 antidepressants failed me and my doc was like oh cool here’s your report and then just handed it to me and said good luck lol. i had to google CYP2C19 myself and then go to a pharmacist who actually knew what it meant. why is this so hard. why does the system make you your own advocate. its 2025 not 1995
S Love
This is exactly the kind of breakthrough that should be standard of care. I’ve seen patients who’ve been on five different SSRIs over five years, each time worse than the last. One test, one conversation with a clinical pharmacist, and suddenly they’re sleeping, eating, and smiling again. It’s not just about drugs-it’s about dignity. You don’t deserve to suffer because your doctor didn’t learn genetics in med school. We need to make this routine, not rare.
Jim Peddle
Of course they’re pushing this. Big Pharma owns the labs, the EHR vendors, the CPIC guidelines. You think they want you to avoid toxic drugs? No. They want you to keep taking drugs-just the right branded ones. And if you’re a slow metabolizer? They’ll just push a higher dose. Or a new combo. Or a new drug with a patent. This isn’t about your health. It’s about profit margins dressed up as science.
Pritesh Mehta
Western medicine thinks it owns biology. But in India, we’ve been using Ayurvedic principles for 5,000 years to match herbs to body types-doshas, prakriti, agni-all of which mirror pharmacogenomics in spirit. We didn’t need a $400 test to know that some people burn through medicine like a candle. Why is it only now considered ‘cutting-edge’ when Western labs replicate what ancient systems understood intuitively? The arrogance of colonial science is breathtaking.
Billy Tiger
Look I’m all for science but if you’re telling me my genes decide if I live or die based on some spit test then why is my VA still prescribing me codeine like its 1998? This is just another excuse to avoid fixing the real problem: doctors don’t listen. They don’t care. They’re too busy chasing quotas. Genetics? Nah. Just give me another script and move on to the next patient
Katie Ring
It’s not about the genes. It’s about who gets to define normal. The FDA, CPIC, the labs-they all operate on a narrow set of data. What about people who are mixed race? Non-binary? Elderly with multiple comorbidities? The algorithm doesn’t care. It just spits out a recommendation based on averages. And if you don’t fit the model? You’re just noise. That’s not medicine. That’s math pretending to be care.
Adarsha Foundation
I’ve worked with patients who were terrified of genetic testing because they feared discrimination. But when they finally understood it wasn’t about predicting disease-it was about avoiding harm-they felt empowered. The real barrier isn’t cost or science. It’s fear. We need more education, more compassion, more space for people to ask questions without judgment. This isn’t just a test. It’s a conversation waiting to happen.
Alex Sherman
Let’s not pretend this is revolutionary. We’ve had pharmacogenomic data for 20 years. The reason it’s not mainstream is because it threatens the status quo. Doctors don’t want to be told what to prescribe. Pharmacies don’t want to slow down filling scripts. Insurance won’t pay unless forced. This isn’t a scientific gap. It’s a power gap. And until we fix that, the test will remain a luxury for the privileged few.
Oliver Myers
I just want to say thank you for writing this. My sister tried 11 different meds for her anxiety before finally getting tested. Turns out she’s a CYP2D6 poor metabolizer-everything made her feel like she was drowning. After switching to bupropion, she went from barely leaving the house to hiking with friends again. It didn’t fix everything, but it gave her back her life. This isn’t just data. It’s hope. Please keep sharing stories like this.
Andy Ruff
You people are naive. You think this is about health? It’s about control. They’re not testing your genes to help you-they’re mapping your biology to sell you more drugs, more supplements, more ‘personalized’ wellness products. Soon, your DNA will be tied to your insurance premiums. Employers will screen for ‘high-risk’ metabolizers. You’ll be labeled ‘non-compliant’ because your liver doesn’t cooperate with Big Pharma’s profit model. This isn’t progress. It’s surveillance with a lab coat.
And don’t tell me about CPIC guidelines-they’re written by consultants who get paid by the same companies that make the drugs. You think they want you to avoid codeine? No. They want you to pay for the expensive alternative. That’s why they’re pushing this so hard. It’s not science. It’s a business model.
And the racial bias? Of course it’s there. The NIH didn’t suddenly start including diverse populations because they cared. They were sued. The data is still garbage for non-Europeans. You think your test result is accurate if your ancestry is 40% African and 60% European? Good luck. The algorithm doesn’t know how to handle that. It just picks the closest white reference sample and calls it a day.
And the cost? $400? That’s a joke. The actual cost of processing the sample is $15. The rest is markup, insurance billing, EHR integration fees, and corporate greed. And who pays? You. The patient. The one who’s already suffering. This isn’t medicine. It’s a pyramid scheme with DNA.
They’ll tell you it’s saving lives. But the real number? Adverse reactions are still rising. Because the system doesn’t change. It just adds another layer of bureaucracy. You get your report. You show your doctor. They shrug. They prescribe anyway. Because they don’t trust it. Or they don’t have time. Or they’re scared of liability. So you’re left with a $400 receipt and no answers.
And then they pat themselves on the back and call it innovation.
It’s not innovation. It’s exploitation dressed in a white coat.
John Concepcion
Bro just got tested and my report says I’m a super fast metabolizer so all my meds get flushed out in 2 hours. So I just started taking 3x the dose. Now I’m a zombie. Thanks genes.

Write a comment