Autoimmune Uveitis: How Steroid-Sparing Therapy Protects Your Vision
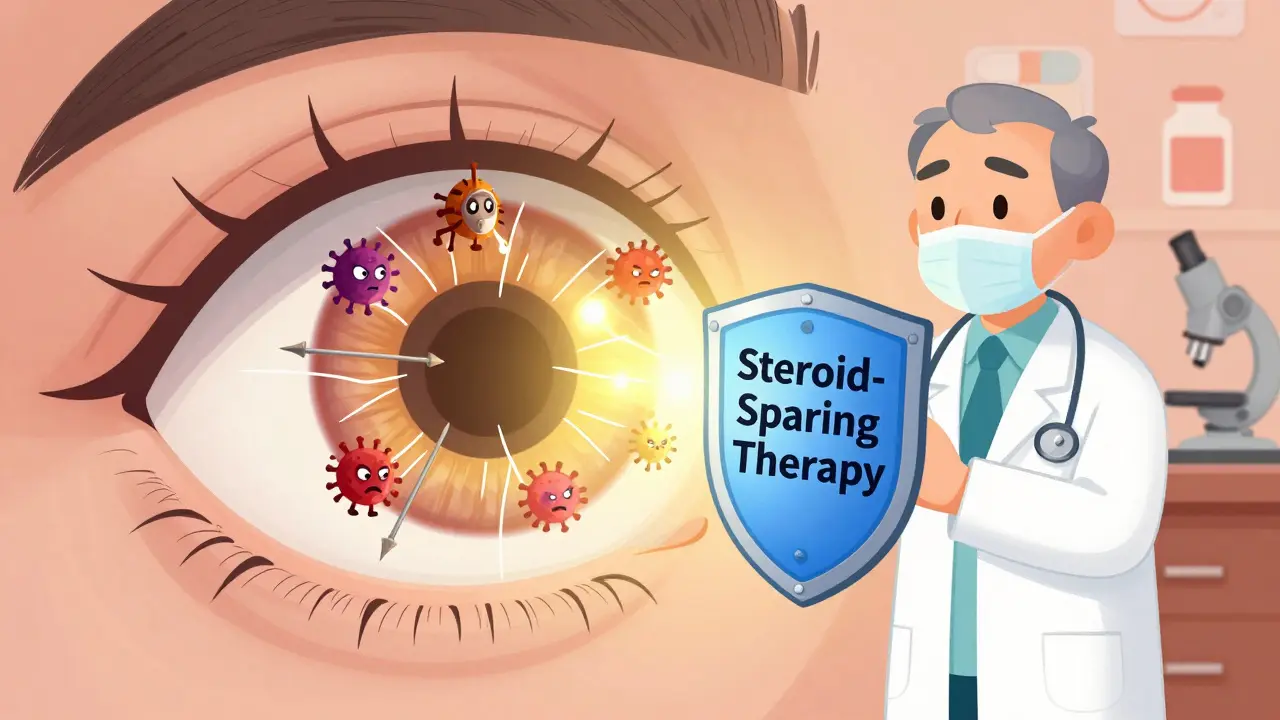
Autoimmune uveitis isn’t just a red eye. It’s a silent threat that can steal your vision if left unchecked. Unlike regular eye irritation, this condition happens when your immune system turns against your own eye tissue-attacking the uvea, the middle layer that feeds the retina and controls pupil size. It doesn’t come with a warning sign. Symptoms can sneak up: blurred vision, light sensitivity, floaters, eye pain. By the time you notice, damage may already be done. And while steroids are the first line of defense, they’re not a long-term solution. That’s where steroid-sparing therapy comes in.
What Exactly Is Autoimmune Uveitis?
Uveitis means inflammation of the uvea. When it’s autoimmune, your body’s defense system mistakes parts of your eye for foreign invaders. This isn’t caused by an infection like bacteria or viruses. It’s internal-your own immune cells attacking your eye. It can strike one eye or both, and it doesn’t care how old you are or how healthy you think you are.
It’s rare-fewer than 200,000 people in the U.S. have it-but that doesn’t make it less dangerous. About half of all autoimmune uveitis cases are tied to other autoimmune diseases. Think ankylosing spondylitis, lupus, Crohn’s disease, psoriasis, or multiple sclerosis. If you have one of these, your risk goes up. And if you have uveitis without a clear cause, doctors will check for these hidden conditions.
Left untreated, uveitis can lead to cataracts, glaucoma, macular edema, retinal detachment, and permanent vision loss. The inflammation doesn’t just hurt-it scars. That’s why early diagnosis and aggressive treatment aren’t optional. They’re life-changing.
Why Steroids Are a Double-Edged Sword
Corticosteroids are the go-to treatment. Eye drops for front-of-the-eye inflammation. Injections near the eye for deeper issues. Pills for widespread or severe cases. They work fast-sometimes in days. That’s why they’re the first step.
But here’s the catch: steroids aren’t harmless. Long-term use causes cataracts, raises eye pressure (leading to glaucoma), weakens bones, spikes blood sugar, causes weight gain, and increases infection risk. For someone with uveitis that keeps coming back, steroid dependence becomes a prison. You’re trading one problem for another.
Doctors know this. That’s why, for anyone with chronic or recurrent uveitis, the goal isn’t just to control inflammation-it’s to get off steroids entirely. That’s where steroid-sparing therapy isn’t just helpful. It’s essential.
Steroid-Sparing Therapy: What It Is and How It Works
Steroid-sparing therapy means using other drugs that calm the immune system so you don’t need high-dose steroids anymore. These aren’t quick fixes. They take weeks or months to kick in. But once they do, they can keep inflammation under control for years-with far fewer side effects.
The most common options include:
- Methotrexate: An old-school immunosuppressant, often used for rheumatoid arthritis. It’s affordable and widely available. Works well for many, but needs regular blood tests to watch for liver or blood cell issues.
- Cyclosporine: Originally developed for organ transplant patients. It’s powerful but can affect kidney function. Requires careful monitoring.
- Adalimumab (Humira): The first FDA-approved biologic specifically for non-infectious uveitis. Approved in 2016, it blocks TNF-alpha, a key inflammatory protein. Studies show it reduces flare-ups by over 60% and cuts steroid use significantly. Many patients go from daily pills to monthly injections-and keep their vision.
- Infliximab: Another TNF blocker, given by IV. Especially effective in children with uveitis, where steroid damage can ruin lifelong vision.
These drugs don’t cure uveitis. But they change the game. They turn a chronic, steroid-dependent condition into a manageable one. For many, it means fewer doctor visits, no more cataract surgeries, and the ability to live without fear of sudden vision loss.

How Doctors Decide Which Therapy to Use
There’s no one-size-fits-all. Treatment depends on three things: where the inflammation is, how bad it is, and what else is going on in your body.
Anterior uveitis (front of the eye) might start with eye drops and a short steroid course. If it comes back, they’ll add methotrexate. Posterior uveitis (back of the eye) is trickier. It often needs injections or pills from the start. If you have lupus or Crohn’s, your rheumatologist and ophthalmologist will team up. That’s key. Uveitis doesn’t live in isolation. It’s a sign of something bigger.
Before starting any steroid-sparing drug, doctors run tests. Blood work. Imaging. Sometimes a fluorescein angiogram to map blood flow in the retina. They need to rule out infections like tuberculosis or herpes-because treating an infection with an immunosuppressant can be deadly.
And timing matters. The NHS and Mayo Clinic both stress: if you have uveitis, see an ophthalmologist within 24 hours. Delaying treatment increases the risk of permanent damage. Steroid-sparing drugs take time. You can’t wait to start them.
What Patients Really Experience
People on steroid-sparing therapy often describe a shift-from fear to control. One patient, a 32-year-old teacher with recurrent uveitis tied to ankylosing spondylitis, went from needing three steroid injections a year to just one every 16 weeks on adalimumab. Her vision stabilized. She stopped gaining weight. Her blood sugar returned to normal.
But it’s not all smooth. Immunosuppressants increase infection risk. You might get more colds. You’ll need vaccines (flu, pneumonia, shingles) before starting. You can’t take live vaccines after. Some feel tired. Others get nausea. Regular blood tests every 4-8 weeks are non-negotiable.
Adherence is a problem. These drugs are expensive. Some require weekly or monthly injections. Others need pills twice a day. If you miss doses, inflammation can flare. That’s why support matters-educating patients, setting reminders, connecting them with nurse navigators.
The payoff? Better quality of life. Fewer surgeries. Less dependence on eye drops that burn. The ability to drive, read, work, and live without the shadow of blindness.

The Future: Personalized Treatment and New Drugs
The field is moving fast. Seven new biologics are in clinical trials targeting different parts of the immune system-interleukin inhibitors, JAK-STAT blockers, and more. These could help people who don’t respond to TNF blockers like Humira.
Researchers are also looking at genetic markers and blood proteins that predict who will respond to which drug. Imagine a simple blood test that tells you: ‘You’re likely to respond to adalimumab, but not methotrexate.’ That’s the future. Personalized medicine isn’t science fiction here-it’s already being tested in uveitis clinics.
Specialized uveitis centers have grown from 15 in 2010 to over 50 in 2023. These aren’t just eye clinics. They’re multidisciplinary hubs where rheumatologists, immunologists, and ophthalmologists sit at the same table. That’s how you get the best outcomes.
When Surgery or Other Treatments Still Help
Even with the best drugs, some damage is done. If you develop cataracts from years of steroids, surgery can restore vision. If pressure builds up in your eye, laser or surgery may be needed for glaucoma. In rare cases, vitrectomy (removing the gel inside the eye) helps clear inflammation.
But these are last resorts. The goal is to prevent them. That’s why steroid-sparing therapy isn’t just a backup plan. It’s the main plan for anyone with chronic uveitis.
What You Should Do Now
If you’ve been diagnosed with uveitis:
- Ask if it’s autoimmune or infectious. This changes everything.
- Ask if you’re a candidate for steroid-sparing therapy. Don’t wait until you’re on high-dose steroids for years.
- Get a rheumatology consult if you have another autoimmune condition.
- Find a uveitis specialist. Not all eye doctors treat this regularly.
- Get vaccinated before starting immunosuppressants.
- Stick to your monitoring schedule. Blood tests aren’t optional.
If you’re on steroids and your uveitis keeps coming back, you’re not failing. You’re just in the wrong treatment phase. Steroid-sparing therapy isn’t a sign of defeat. It’s the next step toward living with your condition-not being ruled by it.
Is autoimmune uveitis curable?
No, autoimmune uveitis isn’t curable. But it can be controlled. With the right steroid-sparing therapy, many people achieve long-term remission-meaning no flare-ups for years. The goal isn’t a cure. It’s protecting your vision and quality of life.
Can I stop steroids right away if I start steroid-sparing therapy?
No. Steroid-sparing drugs take weeks to months to work. Stopping steroids too fast can cause a dangerous flare. Doctors slowly reduce steroid doses while the new drug builds up in your system. This process is carefully monitored.
Are biologics like Humira safe for long-term use?
Yes, for most people. Humira has been used safely for over a decade in uveitis patients. The main risks are increased infections and rare immune-related side effects. Regular check-ups and blood tests make these risks manageable. The benefits-preserving vision and avoiding steroid damage-far outweigh the risks for chronic cases.
What if I can’t afford steroid-sparing drugs?
Many biologics are expensive, but patient assistance programs exist through drug manufacturers and nonprofits. Methotrexate and cyclosporine are much cheaper and still effective for many. Talk to your doctor or a hospital social worker-financial help is often available.
Can uveitis come back even on steroid-sparing therapy?
Yes, it can. But the frequency and severity drop dramatically. Many patients go from 3-4 flares a year to one every 2-3 years. If a flare happens, doctors can adjust the dose or add a short steroid burst-without going back to long-term steroid dependence.
Autoimmune uveitis doesn’t have to mean losing your sight. The tools to protect your vision are here. The key is acting early, thinking long-term, and choosing therapy that doesn’t trade one problem for another. Steroid-sparing treatment isn’t just medical progress. It’s a lifeline.
12 Comments
Lydia H.
Just wanted to say this post saved my vision. I had uveitis for three years on steroids-weight gain, cataracts, the whole nightmare. Started Humira last year. No more flares. No more eye drops burning like acid. I can drive at night again. Thank you for writing this.
Josh Kenna
Same here. I thought I was doomed to be blind by 40. My doc pushed me to try methotrexate after my third flare. Took 10 weeks to kick in, but now I’m on zero steroids. Blood tests suck, but they’re worth it. Also-get the shingles vaccine before starting anything. Learned that the hard way.
Erwin Kodiat
As someone from India who moved to the US for treatment-this is the first time I’ve seen uveitis talked about like a real disease, not just ‘eye redness.’ In my hometown, doctors just gave drops and said ‘rest your eyes.’ I cried reading this. Thank you.
Jackson Doughart
The distinction between infectious and autoimmune uveitis is critical. Too many cases are misdiagnosed as conjunctivitis. The delay in proper intervention is what leads to irreversible damage. This is not hyperbole-it’s clinical reality.
Malikah Rajap
Wait… so… you’re saying… your body is literally attacking your eyes… like… a betrayal?… like… your immune system is a jealous ex?… and Humira is the therapist who says, ‘No, you don’t need to hurt yourself anymore’?… I’m crying. I’m not crying. I’m just… blinking a lot. This is poetry.
sujit paul
Let me tell you something. Big Pharma invented uveitis to sell biologics. Steroids have been used for 70 years. Why suddenly need $20,000 injections? Look at the stock prices of AbbVie. Coincidence? I think not. The truth is hidden behind medical jargon. Trust your body. Eat turmeric. Pray. Avoid eye doctors who speak in acronyms.
Tracy Howard
Canada has universal healthcare. We don’t need to beg for biologics like Americans. We get them. And we don’t have to pay $1000/month just to keep our eyes. So please, stop acting like this is some heroic breakthrough-it’s just basic medicine in a civilized country.
Aman Kumar
Immunosuppressants are a gateway to opportunistic infections. You think you’re saving your vision, but you’re just becoming a walking Petri dish. TB reactivation. Hepatitis B resurgence. Fungal meningitis. The CDC has published 14 case reports of fatal outcomes. You’re trading one death for another. This is not medicine-it’s calculated risk with a glossy brochure.
Jake Rudin
Is the uvea… not just tissue… but a metaphor?… the soul of the eye?… and when the immune system attacks it… is it not the self turning against the self?… the body’s silent scream… that we mistake for mere inflammation?… Perhaps… the cure is not in drugs… but in harmony…
Astha Jain
so uveitis is like when your body gets too stressed and starts yelling at your eyes?? like a bad roommate?? i got it. also methotrexate is cheaper than my coffee subscription lmao
Phil Hillson
This whole post is just a big ad for Humira. I’ve had uveitis for 8 years. I’m on steroids. I’m fine. Why are you making me feel guilty for not taking some expensive biologic? Also, I don’t need a ‘uveitis specialist.’ I have an eye doctor. End of story.
Lewis Yeaple
While the clinical data supporting steroid-sparing agents is robust, one must not overlook the importance of longitudinal monitoring. The 2022 AAO guidelines emphasize baseline serologic screening for latent infections prior to initiation of biologics. Furthermore, adherence rates below 85% correlate strongly with increased flare frequency. This is not anecdotal-it is evidence-based.




Write a comment