Generic Drugs: What They Are, Why They Save Money, and How to Use Them Safely
When you hear generic drugs, medications that contain the same active ingredients as brand-name pills but are sold under their chemical name. Also known as generic medications, they are approved by the FDA to work exactly like the original—same dose, same effect, same safety profile. Yet most people still pay more than they need to because they don’t know the difference between a brand name and a generic.
Here’s the truth: brand name drugs, medications developed and marketed by pharmaceutical companies under a patent. Also known as originator drugs, they cost hundreds or even thousands of dollars because the company recoups research and marketing expenses. Once the patent expires, other manufacturers can produce the same drug using the same formula. That’s when FDA approved generics, copies of brand-name drugs that meet strict quality, strength, and purity standards set by the U.S. Food and Drug Administration. Also known as generic equivalents, they are tested to ensure they deliver the same results in your body. No extra ingredients. No hidden changes. Just the same medicine at 80% less cost.
But not all online pharmacies are honest. That’s why counterfeit drugs, fake medications that may contain no active ingredient, wrong dose, or dangerous contaminants. Also known as fake pills, they flood unregulated websites and can cause serious harm. You’ll find posts here that show you exactly how to verify a pharmacy using NABP Verify and VIPPS badges—so you never accidentally buy a placebo or poison. These aren’t theoretical warnings. People have ended up in the ER because they bought "generic Lipitor" or "generic Viagra" from a site that looked real but wasn’t.
What you’ll find below isn’t just a list of articles. It’s a practical toolkit. You’ll learn how generic drugs like atorvastatin (Lipitor), sildenafil (Viagra), and ibuprofen (Motrin) compare to their brand-name versions. You’ll see how biosimilars—complex versions of biologic drugs—are cutting costs for cancer and autoimmune treatments. You’ll get real advice on when a drug holiday makes sense and how your genes might affect how your body handles a generic pill. You’ll even find guides on what to eat while taking chemotherapy drugs like chlorambucil, or how to use an inhaler correctly so your asthma medication actually works.
This isn’t about cutting corners. It’s about cutting costs without cutting corners. Generic drugs have been saving patients billions for decades. But only if you know how to get them safely and use them right. Below, you’ll find everything you need to stop overpaying—and start getting the full benefit of the medicine you’re already prescribed.
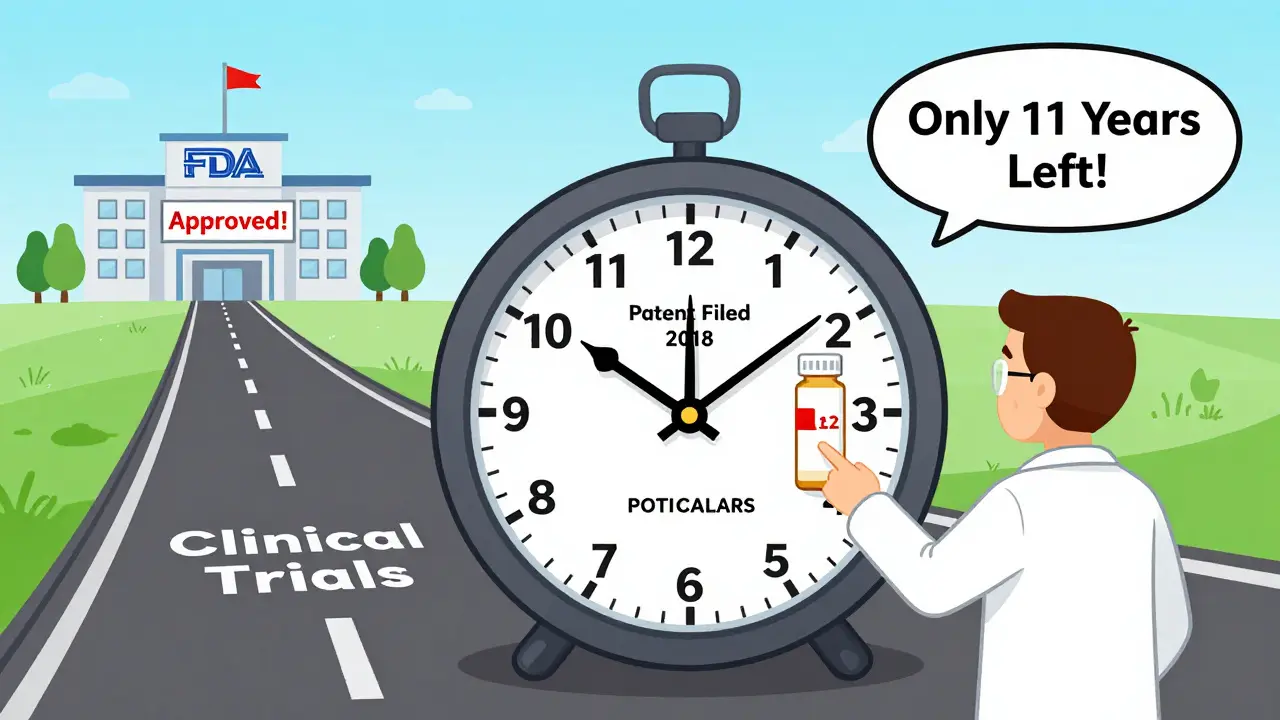
When Do Drug Patents Expire? Understanding the 20-Year Term and Real-World Timelines
Drug patents last 20 years from filing, but most drugs lose exclusivity in just 7-12 years due to long development times. Learn how patent extensions, regulatory exclusivity, and legal tactics shape when generics enter the market.
Read MoreHow to Compare New Prescription Labels with Your Old Medication for Safety
Learn how to safely compare your new prescription label with your old one to avoid dangerous medication errors. Know what to check, how to spot mismatches, and when to call your pharmacist.
Read MoreWhy Insurers Prefer Generic Drugs: How Formularies Control Costs and Guide Prescriptions
Insurers prefer generic drugs because they’re clinically identical but cost up to 95% less. Preferred generic lists guide patients toward cheaper options, saving billions - but not without trade-offs. Learn how formularies work and how to save money on prescriptions.
Read MoreForeign Manufacturing of Generics: How the FDA Oversees Drug Quality Abroad
The FDA has ended its double standard in inspecting foreign generic drug factories. Unannounced inspections are now the norm, forcing global manufacturers to meet the same quality standards as U.S. plants. Here’s how it works-and what it means for your medications.
Read MoreCommon Pharmacist Concerns About Generic Substitution: What Really Happens Behind the Counter
Pharmacists support generic substitution to cut costs and improve access, but face major hurdles from patient mistrust, lack of prescriber communication, and complex clinical cases. Here’s what really happens behind the counter.
Read MoreHow to Handle Insurance Requirements for Generic Substitution
Learn how insurance companies enforce generic drug substitution, when you can fight back, and how to get brand-name medications covered - with real rules, state laws, and patient experiences.
Read MoreFDA Bioequivalence Standards for NTI Drugs: What You Need to Know
The FDA enforces stricter bioequivalence standards for narrow therapeutic index (NTI) drugs like warfarin, phenytoin, and digoxin to prevent dangerous dosing variations. Learn how these rules differ from standard generics and why they matter for patient safety.
Read MoreAntitrust Issues in Generic Substitution: How Drug Companies Block Cheaper Alternatives
Drug companies are using legal loopholes to block cheaper generic drugs by withdrawing original formulations and launching minor reformulations. This tactic, called product hopping, costs patients and taxpayers billions each year.
Read MoreBridging Studies for NTI Generics: Ensuring Safety and Efficacy
NTI generics require stricter bioequivalence studies than standard generics to ensure safety. Learn how bridging studies, FDA guidelines, and complex trial designs protect patients using critical medications like warfarin and levothyroxine.
Read More